 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H25NO |
| Molar mass | 247.382 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Picenadol (LY-97435) is a 4-phenylpiperidine derivative that is an opioid analgesic drug developed by Eli Lilly in the 1970s.
Picenadol is an effective analgesic with similar efficacy to pethidine (meperidine). It has been investigated for some applications such as obstetrics and dentistry, but never commercialised.
It is unusual in that one enantiomer is a pure μ-opioid agonist, while the other is an antagonist. The (3S,4R) isomer is the agonist, while (3R,4S) is antagonist. This means that the racemic mix of the two enantiomers is a mixed agonist-antagonist, with relatively low abuse potential, and little of the κ-opioid activity that tends to cause problems with other opioid mixed agonist-antagonists such as pentazocine.
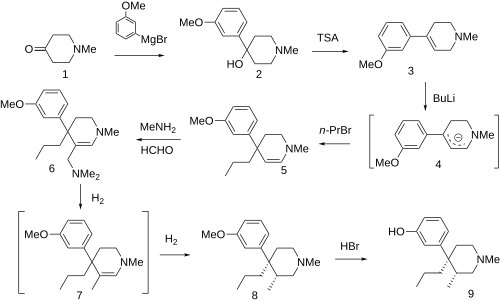
Synthesis

See also
References
- US 4081450, Zimmerman DM, "1,3,4-Trisubstituted-4-arylpiperidines and their preparation", issued 28 April 1978, assigned to Eli Lilly & Company
- Sherline DM (October 1983). "Picenadol (LY 150720) compared with meperidine and placebo for relief of post-cesarean section pain: a randomized double-blind study". American Journal of Obstetrics and Gynecology. 147 (4): 404–6. doi:10.1016/s0002-9378(16)32234-7. PMID 6624809.
- Goldstein DJ, Brunelle RL, George RE, Cooper SA, Desjardins PJ, Gaston GW, Jeffers GE, Gallegos LT, Reynolds DC (1994). "Picenadol in a large multicenter dental pain study". Pharmacotherapy. 14 (1): 54–9. doi:10.1002/j.1875-9114.1994.tb02789.x. PMID 8159602. S2CID 24644644.
- Leander JD, Zimmerman DM (December 1983). "Effects of picenadol and its agonist and antagonist isomers on schedule-controlled behavior". The Journal of Pharmacology and Experimental Therapeutics. 227 (3): 671–5. PMID 6655562.
- Froimowitz M, Cody V. Absolute configurations and conformations of the opioid agonist and antagonist enantiomers of picenadol. Chirality. 1995;7(7):518-25.
- Zimmerman DM, Smits SE, Hynes MD, Cantrell BE, Leander JD, Mendelsohn LG, Nickander R (February 1985). "Picenadol". Drug and Alcohol Dependence. 14 (3–4): 381–401. doi:10.1016/0376-8716(85)90069-9. PMID 2986931.
- US 4499274, Feth G, Mills JE, "Process for preparation of substituted formamidine and substituted N-iminomethyl piperidine", published 1985-02-12, assigned to McNeil Lab Inc.
- Martinelli MJ, Peterson BC (1990). "A concise, stereoselective synthesis of picenadol". Tetrahedron Letters. 31 (38): 5401–5404. doi:10.1016/S0040-4039(00)97857-2.
This analgesic-related article is a stub. You can help Misplaced Pages by expanding it. |