| |
| |
| Names | |
|---|---|
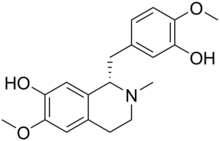
| IUPAC name 3,10-Dimethoxy-8,8a-secoberbine-2,9-diol | |
| Systematic IUPAC name (1S)-1--6-methoxy-2-methyl-1,2,3,4-tetrahydroisoquinolin-7-ol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.920 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C19H23NO4 |
| Molar mass | 329.396 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Reticuline is an alkaloid found in opium and a variety of plants including Lindera aggregata, Annona squamosa, and Ocotea fasciculata (also known as Ocotea duckei).
Experiments in rodents suggest reticulije possesses potent central nervous system depressing effects. It is the precursor of morphine and many other alkaloids. It is also toxic to dopaminergic neurons causing a form of atypical parkinsonism known as Guadeloupean Parkinsonism.
Metabolism
3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase uses S-adenosyl methionine and 3'-hydroxy-N-methyl-(S)-coclaurine to produce S-adenosylhomocysteine and (S)-reticuline.
Reticuline oxidase uses (S)-reticuline and O2 to produce (S)-scoulerine and H2O2.
Salutaridine synthase uses (R)-reticuline, NADPH, H, and O2 to produce salutaridine, NADP, and H2O. Salutaridine can then be transformed progressively to thebaine, oripavine, and morphine.
1,2-dehydroreticulinium reductase (NADPH) uses (R)-reticuline and NADP to produce 1,2-dehydroreticulinium, NADPH, and H.
References
- Han, Zheng; Zheng, Yunliang; Chen, Na; Luan, Lianjun; Zhou, Changxin; Gan, Lishe; Wu, Yongjiang (2008). "Simultaneous determination of four alkaloids in Lindera aggregata by ultra-high-pressure liquid chromatography–tandem mass spectrometry". Journal of Chromatography A. 1212 (1–2): 76–81. doi:10.1016/j.chroma.2008.10.017. PMID 18951552.
- Dholvitayakhun, Achara; Trachoo, Nathanon; Sakee, Uthai; et al. (2013). "Potential applications for Annona squamosa leaf extract in the treatment and prevention of foodborne bacterial disease". Natural Product Communications. 8 (3): 385–388. doi:10.1177/1934578X1300800327. PMID 23678817.
- ^ de Morais, Liana Clébia Soares Lima; Barbosa-Filho, José Maria; de Almeida, Reinaldo Nóbrega (1998). "Central depressant effects of reticuline extracted from Ocotea duckei in rats and mice". Journal of Ethnopharmacology. 62 (1): 57–61. doi:10.1016/S0378-8741(98)00044-0. PMID 9720612.
- Bradley's neurology in clinical practice. Daroff, Robert B.,, Jankovic, Joseph,, Mazziotta, John C.,, Pomeroy, Scott Loren,, Bradley, W. G. (Walter George) (Seventh ed.). London. 2015-10-25. ISBN 9780323339162. OCLC 932031625.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link)
External links
![]() The dictionary definition of reticuline at Wiktionary
The dictionary definition of reticuline at Wiktionary