
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name N,N′-(Ethane-1,2-diyl)bis(N-acetylacetamide) | |||
| Other names TAED, N,N′-ethylenebis(diacetamide) | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.031.009 | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C10H16N2O4 | ||
| Molar mass | 228.248 g·mol | ||
| Appearance | Colorless solid | ||
| Density | 0.9 | ||
| Melting point | 149 to 154 °C (300 to 309 °F; 422 to 427 K) | ||
| Solubility in water | 0.2 g/L @ 20 °C | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Tetraacetylethylenediamine, commonly abbreviated as TAED, is an organic compound with the formula (CH3C(O))2NCH2CH2N(C(O)CH3)2. This white solid is commonly used as a bleach activator in laundry detergents and for paper pulp. It is produced by acetylation of ethylenediamine.
Use and mechanism of action
TAED is an important component of laundry detergents that use "active oxygen" bleaching agents. Active oxygen bleaching agents include sodium perborate, sodium percarbonate, sodium perphosphate, sodium persulfate, and urea peroxide. These compounds release hydrogen peroxide during the wash cycle, but the release of hydrogen peroxide is low when these compounds are used in temperatures below 45 °C (113 °F). TAED and hydrogen peroxide react to form peroxyacetic acid, a more efficient bleach, allowing lower temperature wash cycles, around 40 °C (104 °F). TAED was first used in a commercial laundry detergent in 1978 (Skip by Unilever). Currently, TAED is the main bleach activator used in European laundry detergents and has an estimated annual consumption of 75 kt.
Perhydrolysis
TAED reacts with alkaline peroxide via the process called perhydrolysis releasing of peracetic acid. The first perhydrolysis gives triacetylethylenediamine (TriAED) and the second gives diacetylethylenediamine (DAED):

|
TAED typically provides only two equivalents of peracetic acid, although four are theoretically possible. Competing with perhydrolysis, TAED also undergoes some hydrolysis, which is an unproductive pathway.
Preparation
TAED is prepared in a two-stage process from ethylenediamine and acetic anhydride. The process is nearly quantitative.
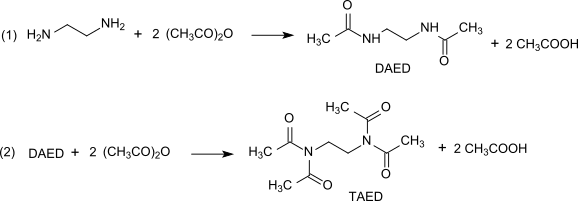
|
Properties
Powdered TAED is stabilized by granulation with the aid of the sodium salt of carboxymethylcellulose (Na-CMC), which are sometimes additionally coated blue or green. Despite the relatively low solubility of TAED in cool water, (1 g/L at 20 °C), the granulate dissolves rapidly in the washing liquor.
The peroxyacetic acid formed has bactericidal, virucidal and fungicidal properties, thereby enabling TAED with percarbonate to disinfect and deodorize.
Ecology
Triacetylethylenediamine is mostly non-toxic and easily biodegradable. TAED and its byproduct DAED have low aquatic ecotoxicity. Triacetylethylenediamine shows a very low toxicity in all exposure routes, is practically non-irritating effect on skin and eye, and does not give any indication of skin sensitization. It is not mutagenic and not teratogenic. TAED, TriAED and DAED are all completely biodegradable and substantially removed during wastewater treatment.
References
- Smulders E.; von Rybinski W.; Sung E.; Rähse W.; Steber J.; Wiebel F.; Nordskog A. (2002). "Laundry Detergents". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH.
- Reinhardt, G.; Borchers, G. (2009). "Application of Bleaching Detergent Formulations". In Zoller, Uri (ed.). Handbook of Detergents Part E: Applications. USA: CRC Press. ISBN 9781574447576.
- D. Martin Davies and Michael E. Deary "Kinetics of the hydrolysis and perhydrolysis of tetraacetylethylenediamine, a peroxide bleach activator" J. Chem. Soc., Perkin Trans. 2, 1991, pages 1549 - 1552. doi:10.1039/P29910001549.
- Farr, J. P.; Smith, W. L.; Steichen,, D. S. (1992). "Bleaching Agents". Kirk-Othmer Encyclopedia of Chemical Technology Vol. 4 (4th ed.). John Wiley & Sons, Inc. pp. 271–299.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Hauthal, G. H., Schmidt, H., Scholz, J., Hofmann, J. and Pritzkow W. (1990). "Studies concerning the mechanism of bleach activation". Tenside Surf. Det. 27 (3). doi:10.1515/tsd-1990-270314. S2CID 235325458.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hofmann, J., Just, G., Pritzkow, W. and Schmidt, H. (1992). "Bleaching activators and the mechanism of bleaching activation". Journal für Praktische Chemie/Chemiker-Zeitung. 334 (4): 293–297. doi:10.1002/prac.19923340402.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Europäische Patentanmeldung EPA 004 919, Verfahren zur Herstellung von N,N,N’,N’-Tetraacetyl-ethylendiamin, Erfinder: G. Müller-Schiedmayer, R. Aigner, Anmelder: Hoechst AG, veröffentlicht am 31. Oktober 1979
- Europäische Patentanmeldung EPA 0 051 739 A1, Verfahren zur Herstellung von N,N,N’,N’-Tetraacetylethylendiamin, Erfinder: W. Köhler et al., Anmelder: BASF AG, veröffentlicht am 19. Mai 1982
- Europäisches Patent EP 0 238 958 B1, Verfahren zur Reinigung von Tetraacetylethylendiamin, Erfinder: K. Köster, F.-J. Carduck, Anmelder: Henkel KG aA, veröffentlicht am 12. Juni 1991
- US-Patent US 5,100,576, Process for the preparation of a readily soluble bleach activator granulate with a long shelf life, Erfinder: J. Cramer et al., Anmelder: Hoechst AG, erteilt am 31. März 1992.
- Clariant Surfactant Division: The Clean and Clever Way of Bleaching - PERACTIVE Archived 2013-07-17 at the Wayback Machine (PDF; 865 kB), 08.99
- HERA Human & Environmental Risk Assessment on ingredients of European household cleaning products: Tetraacetylethylenediamine (TAED), CAS 10543-57-4 (PDF; 666 kB), Draft, DECEMBER 2002.
- Gilbert, P. A. (1992). "TAED-Tetraacetylethylenediamine". In de Oude, N. T. (ed.). The Handbook of Environmental Chemistry, Vol. 3 Part F Antropogenic Compounds: Detergents. Berlin: Springer-Verlag. ISBN 354053797X.
