 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
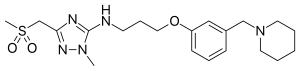
| Formula | C20H31N5O3S |
| Molar mass | 421.56 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Sufotidine (INN, USAN, codenamed AH25352) is a long-acting competitive H2 receptor antagonist which was under development as an antiulcerant by Glaxo (now GlaxoSmithKline). It was planned to be a follow-up compound to ranitidine (Zantac). When taken in doses of 600 mg twice daily it induced virtually 24-hour gastric anacidity thus closely resembling the antisecretory effect of the proton pump inhibitor omeprazole. Its development was terminated in 1989 from phase III clinical trials based on the appearance of carcinoid tumors in long-term toxicity testing in rodents.
Synthesis

See also
- Lavoltidine (previously known as loxtidine) — a similar compound in which methylsulfone group is replaced with hydroxyl
References
- "International Nonproprietary Names for Pharmaceutical Substances. Supplement to WHO Chronicle, 1986, Vol. 40, No. 6. Recommended International Nonproprietary Names (Rec. INN): List 26" (PDF). World Health Organization. p. 9. Retrieved 12 January 2016.
- "Drug Profile: Sufotidine". AdisInsight. Springer International Publishing AG. Retrieved 12 January 2016.
- "Glaxo Plans to Follow Up Zantac with Long-acting Sufotidine; H2 Antagonist Is One of Seven Drugs Targeted for Worldwide Marketing Applications in 1988–90". Pharma & MedTech Business Intelligence. Informa Business Intelligence, Inc., an Informa Company. November 30, 1987. Retrieved 12 January 2016.
- Smith JT, Pounder RE (March 1990). "Sufotidine 600 mg bd virtually eliminates 24 hour intragastric acidity in duodenal ulcer subjects". Gut. 31 (3): 291–3. doi:10.1136/gut.31.3.291. PMC 1378269. PMID 1969833.
- Fiorucci S, Santucci L, Farroni F, Pelli MA, Morelli A (October 1989). "Effect of omeprazole on gastroesophageal reflux in Barrett's esophagus". The American Journal of Gastroenterology. 84 (10): 1263–7. PMID 2801676.
- Ganellin CR, Triggle DJ, eds. (1999). Dictionary of Pharmacological Agents (1st ed.). London: Chapman & Hall. ISBN 9780412466304.
- Lawton G, Witty DR, eds. (2011). Progress in Medicinal Chemistry. Vol. 50 (1st ed.). London: Academic. p. 36. ISBN 978-0-12-381290-2.
| Drugs for peptic ulcer and GERD/GORD (A02B) | |
|---|---|
| H2 antagonists ("-tidine") | |
| Prostaglandins (E)/ analogues ("-prost-") | |
| Proton-pump inhibitors ("-prazole") | |
| Potassium-competitive acid blockers ("-prazan") | |
| Others | |
| Combinations | |
| |