 | |
 | |
| Clinical data | |
|---|---|
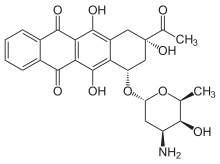
| Other names | 9-acetyl-7-(4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl)oxy-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a691004 |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 97% |
| Elimination half-life | 22 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H27NO9 |
| Molar mass | 497.500 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Idarubicin /ˌaɪdəˈruːbɪsɪn/ or 4-demethoxydaunorubicin is an anthracycline antileukemic drug. It inserts itself into DNA and prevents DNA unwinding by interfering with the enzyme topoisomerase II. It is an analog of daunorubicin, but the absence of a methoxy group increases its fat solubility and cellular uptake. Similar to other anthracyclines, it also induces histone eviction from chromatin.
It belongs to the family of drugs called antitumor antibiotics.
It is currently combined with cytosine arabinoside as a first line treatment of acute myeloid leukemia.
It is used for treatment of acute lymphoblastic leukemia and chronic myelogenous leukemia in blast crisis.
It is distributed under the trade names Zavedos (UK) and Idamycin (USA).
Side effects
Diarrhea, stomach cramps, nausea and vomiting are common among patients treated with idarubicin.
References
- Miller JP, Stoodley RJ (2013). "Studies directed towards anthracyclinone syntheses: The use of d-glucose as a chiral auxiliary in asymmetric Diels–Alder reactions". J. Saudi Chem. Soc. 17: 29–42. doi:10.1016/j.jscs.2011.02.019.
- "Idamycin Package insert" (PDF). Pfizer. January 2006.
- Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, et al. (2013). "Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin". Nature Communications. 4: 1908. Bibcode:2013NatCo...4.1908P. doi:10.1038/ncomms2921. PMC 3674280. PMID 23715267.
- Arwanih EY, Louisa M, Rinaldi I, Wanandi SI (December 2022). "Resistance Mechanism of Acute Myeloid Leukemia Cells Against Daunorubicin and Cytarabine: A Literature Review". Cureus. 14 (12): e33165. doi:10.7759/cureus.33165. PMC 9885730. PMID 36726936.
- Katzung BG (2017-11-30). Basic & clinical pharmacology. McGraw-Hill Education. ISBN 9781259641152. OCLC 1009849139.
- "Idarubicin Side Effects: Common, Severe, Long Term". Drugs.com. Retrieved 2019-06-21.
External links
- Idarubicin bound to proteins in the PDB
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |