 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.234 |
| Chemical and physical data | |
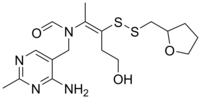
| Formula | C17H26N4O3S2 |
| Molar mass | 398.54 g·mol |
| 3D model (JSmol) | |
SMILES
| |
Fursultiamine (INN; chemical name thiamine tetrahydrofurfuryl disulfide or TTFD; brand names Adventan, Alinamin-F, Benlipoid, Bevitol Lipophil, Judolor, Lipothiamine) is a medication and vitamin used to treat thiamine deficiency. Chemically, it is a disulfide derivative of thiamine and is similar in structure to allithiamine.
It was synthesized in Japan in the 1960s from allithiamine for the purpose of developing forms of thiamine with improved lipophilicity for treating vitamin B1 deficiency (i.e., beriberi), It was subsequently commercialized not only in Japan but also in Spain, Austria, Germany, and the United States.
See also
References
- ^ Lonsdale D (September 2004). "Thiamine tetrahydrofurfuryl disulfide: a little known therapeutic agent". Medical Science Monitor. 10 (9): RA199–203. PMID 15328496.
- Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. p. 1932. ISBN 3-88763-075-0.
Further reading
- Lonsdale D (September 2004). "Thiamine tetrahydrofurfuryl disulfide: a little known therapeutic agent". Medical Science Monitor. 10 (9): RA199–203. PMID 15328496.
| Vitamins (A11) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fat soluble |
| ||||||||
| Water soluble |
| ||||||||
| Combinations | |||||||||
| |||||||||