| |

| |
| Names | |
|---|---|
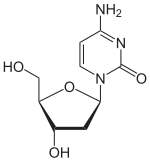
| IUPAC name 2′-Deoxycytidine | |
| Systematic IUPAC name 4-Amino-1-pyrimidin-2(1H)-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.231 |
| MeSH | Deoxycytidine |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H13N3O4 |
| Molar mass | 227.217 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Deoxycytidine is a deoxyribonucleoside, a component of deoxyribonucleic acid. It is similar to the ribonucleoside cytidine, but with one hydroxyl group removed from the C2' position.
Deoxycytidine can be phosphorylated at C5' of the deoxyribose by deoxycytidine kinase, converting it to deoxycytidine monophosphate (dCMP), a DNA precursor. dCMP can be converted to dUMP and dTMP.
It can also be used as a precursor for 5-aza-2′-deoxycytidine, a treatment for MDS patients. This compound slows the cell cycle by interfering with the methylation of the P15/INK4B gene, increasing the expression of P15/INK4B protein which subdues the transformation of MDS to leukemia.
Deoxycytidine can also serve as a biomarker for tumor diagnosis. Deoxycytidine can be used as a biomarker for breast cancer patients and healthy individuals. 5-(Hydroxymethyl)-2′-deoxycytidine (5-hmdC), 5-(formyl)-2′-deoxycytidine (5-fodC), and 5-(carboxyl)-2′-deoxycytidine (5-cadC) are intermediates in the DNA demethylation pathway and can act as biomarkers. 5-hmdC levels were significantly reduced in urine samples of breast cancer patients, while 5-fodC and 5-cadC levels were elevated.
References
- Staub M, Eriksson S (2006). "The Role of Deoxycytidine Kinase in DNA Synthesis and Nucleoside Analog Activation". In Peters GJ (ed.). Deoxynucleoside Analogs In Cancer Therapy. Cancer Drug Discovery and Development. Humana Press. pp. 29–52. doi:10.1007/978-1-59745-148-2_2. ISBN 978-1-59745-148-2.
- Kim KW, Roh JK, Wee HJ, Kim C (2016). "Molecular Targeted Anticancer Drugs". In Kim KW, Roh JK, Wee HJ, Kim C (eds.). Cancer Drug Discovery: Science and History. Springer Netherlands. pp. 175–238. doi:10.1007/978-94-024-0844-7_9. ISBN 978-94-024-0844-7.
- Guo, Mengzhe; Zhang, Liyan; Du, Yan; Du, Wencheng; Liu, Dantong; Guo, Cheng; Pan, Yuanjiang; Tang, Daoquan (2018-03-20). "Enrichment and Quantitative Determination of 5-(Hydroxymethyl)-2′-deoxycytidine, 5-(Formyl)-2′-deoxycytidine, and 5-(Carboxyl)-2′-deoxycytidine in Human Urine of Breast Cancer Patients by Magnetic Hyper-Cross-Linked Microporous Polymers Based on Polyionic Liquid". Analytical Chemistry. 90 (6): 3906–3913. doi:10.1021/acs.analchem.7b04755. ISSN 0003-2700. PMID 29316399.
| Nucleic acid constituents | |||||||
|---|---|---|---|---|---|---|---|
| Nucleobase | |||||||
| Nucleoside |
| ||||||
| Nucleotide (Nucleoside monophosphate) |
| ||||||
| Nucleoside diphosphate | |||||||
| Nucleoside triphosphate | |||||||