 | |
| Clinical data | |
|---|---|
| Trade names | Almarl |
| Other names | S-596 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral (tablets) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 2 hours |
| Elimination half-life | 10 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
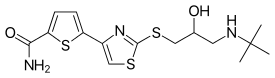
| Formula | C15H21N3O2S3 |
| Molar mass | 371.53 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Arotinolol (INN, marketed under the tradename Almarl) is a medication in the class of mixed alpha/beta blockers. It also acts as a β3 receptor agonist. A 1979 publication suggests arotinolol as having first been described in the scientific literature by Sumitomo Chemical as "β-adrenergic blocking, antiarrhythmic compound S-596".
Medical uses
It is used in the treatment of high blood pressure and essential tremor. Recommended dosage is 10 to 30 mg per day.
References
- Zhao J, Golozoubova V, Cannon B, Nedergaard J (July 2001). "Arotinolol is a weak partial agonist on beta 3-adrenergic receptors in brown adipocytes". Canadian Journal of Physiology and Pharmacology. 79 (7): 585–593. doi:10.1139/cjpp-79-7-585. PMID 11478592.

- Takahashi H, Yoshida T, Nishimura M, Nakanishi T, Kondo M, Yoshimura M (September 1992). "Beta-3 adrenergic agonist, BRL-26830A, and alpha/beta blocker, arotinolol, markedly increase regional blood flow in the brown adipose tissue in anesthetized rats". Japanese Circulation Journal. 56 (9): 936–942. doi:10.1253/jcj.56.936. PMID 1383578.
- Hara Y, Sato E, Miyagishi A, Aono S, Nakatani H (1979). "新しいβ-受容体遮断薬,dl-2-(3'-t-Butylamino-2'-hydroxypropylthio)-4-(5'-carbamoyl-2'-thienyl)-thiazole hydrochloride (S-596) の薬理作用" [Pharmacological properties of dl-2-(3'-t-butylamino-2'-hydroxypropylthio)-4-(5'-carbamoyl-2'-thienyl)thiazole hydrochloride (S-596), a new β-adrenergic blocking agent]. Folia Pharmacologica Japonica (English abstract) (in Japanese). 75 (7): 707–720. doi:10.1254/fpj.75.707. ISSN 1347-8397.

- Wu H, Zhang Y, Huang J, Zhang Y, Liu G, Sun N, et al. (September 2001). "Clinical trial of arotinolol in the treatment of hypertension: dippers vs. non-dippers" (PDF). Hypertension Research. 24 (5): 605–610. doi:10.1291/hypres.24.605. PMID 11675958.

- Lee KS, Kim JS, Kim JW, Lee WY, Jeon BS, Kim D (August 2003). "A multicenter randomized crossover multiple-dose comparison study of arotinolol and propranolol in essential tremor". Parkinsonism & Related Disorders. 9 (6): 341–347. doi:10.1016/S1353-8020(03)00029-4. PMID 12853233.

- "Almarl (アルマール) Arotinolol HCl Tablets. Full Prescribing Information" (PDF). Sumitomo Dainippon Pharma Co., Ltd. Archived from the original (PDF) on 7 May 2012. Retrieved 6 March 2016.
External links
- (in Japanese) Almarl Full Prescribing Information. Revised November 2009 Sumitomo Dainippon Pharma Co., Ltd.
- (in Japanese) Official Sumitomo Dainippon Pharma Website Archived 2020-07-01 at the Wayback Machine
- "Arotinolol Hydrochloride". Drug Monograph. Drug Information Research Institute. Archived from the original on 2011-07-22.
| Beta blockers (C07) | |
|---|---|
| β, non-selective | |
| β1-selective | |
| β2-selective | |
| α1- + β-selective | |
| |