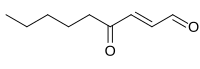
| |
| Names | |
|---|---|
| Preferred IUPAC name (2E)-4-Oxonon-2-enal | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 4963551 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H14O2 |
| Molar mass | 154.209 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
4-Oxo-2-nonenal is a lipid peroxidation product that can structurally alter proteins and induce α-synuclein oligomers.
References
- Näsström, Thomas; Fagerqvist, Therese; Barbu, Mikael; et al. (2011). "The lipid peroxidation products 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote the formation of α-synuclein oligomers with distinct biochemical, morphological, and functional properties". Free Radical Biology and Medicine. 50 (3): 428–437. doi:10.1016/j.freeradbiomed.2010.11.027. PMID 21130160.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |